相关热词搜索: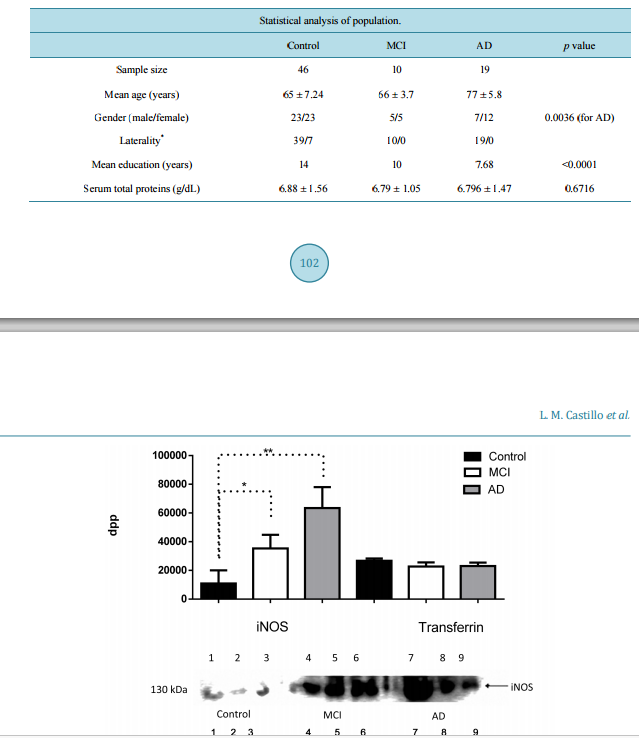 IL-1β, TNF-α and Sambucus nigra Reactive Serum Proteins as Biomarkers of Mild Cognitive Impairment and Alzheimer Disease Progression Luis Manuel Castillo1, Enrique Moreno1, Yaneth Rodríguez-Agudelo2, Mireya Chávez-Oliveros2, Zoila Trujillo3, Blanca Espinosa4, Emma Rodriguez-Maldonado5, Luis Felipe Montaño6, JorgeGuevara1 1 Departamento de Bioquímica, Facultad de Medicina, Universidad Nacional Autónoma de México, México City, México 2 Departamento de Neuropsicología, Instituto Nacional de Neurología y Neurocirugía, México City, México 3 Geriatra Instituto Nacional de Neurología y Neurocirugía, México City, México 4 Laboratorio de Bioquímica, Instituto Nacional de Enfermedades Respiratorias, México City, México 5 Departamento de Fisiología, Instituto Nacional de Cardiología, México City, México 6 Departamento de Biología Celular y Tisular, Facultad de Medicina, Universidad Nacional Autónoma de México, México City, México Received 14 October 2015; accepted 12 December 2015; published 15 December 2015 Copyright © 2015 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Amyloid-β (Aβ) can induce a chronic inflammatory immune response that is associated, amongst many others, to abnormal glycosylation, inducible nitric oxide synthase (iNOS) and nitric oxide (NO). The relation between development of Mild Cognitive Impairment (MCI) and Alzheimer’s disease progression and these serum markers has not been evaluated. Serum levels of iNOs, NO, TNF-α, IL-1β, IL-6, IL-8, IL-10, and IL-12 are determined with commercially available kits. Sialylation of albumin-free serum patterns is determined by Western blot analysis with Sambucus nigra (specific for sialic acid attached to terminal galactose in α2,6 linkage) lectin. Apolipoprotein E (ApoE) haplotype is determined by Western blot using specific anti-ApoE 2, 3 or 4 antibodies. A mini-mental state examination (MMSE) test is also performed in the 10 MCI patients, 19 Alzheimer’s disease (AD) patients and 46 healthy age-matched controls evaluated. The results show an increase of iNOS in MCI and AD but significantly higher NO concentrations are only found in MCI patients. TNF-α and IL-1β concentrations are the only significantly increased cytokines in MCI patients; no differences between control and MCI or AD patients are found in regard to the other cy- L. M. Castillo et al. 100 tokines. An abnormal MMSE test result only correlates with a decrease in serum NO concentration in MCI patients. The terminal sialic acid linkage pattern of serum proteins also shows highly significant differences between MCI and AD patient. ApoE3/4 or 4/4 haplotypes are characteristic of MCI and AD patients. Our results imply that increased serum TNF-α, IL-1β, iNOS, NO and alterations of serum proteins glycosylation patterns in adult inpiduals with an abnormal MMSE test may serve as an early biomarker of MCI and AD development. Keywords Alzheimer Disease, Mild Cognitive Impairment, Nitric Oxide, Inflammatory Cytokines, Glycosylation, Apolipoprotein E4 1. Introduction The majority of pathology alterations in Alzheimer’s disease (AD) have been associated with amyloid-β (Aβ) toxicity [1]. Aβ can activate the microglia and macrophages [2], both of which become amyloid presenting cells to T cells of extra cerebral origin [3]; this in turn favors the local liberation of pro-inflammatory interleukins and reactive oxygen species (ROS) [4], as well as modifications of the post-translation processes of proteins including glycosylation [5]. This modification is not exclusive of immune cells as in vitro activation of endothelial cells exposed to the Aβ peptide has been shown [6]. Brain sections of AD patients express pro-inflammatory interleukins, such as: Interleukin-1 β (IL-1β), Interleukin-6 (IL-6), Interleukin-8 (IL-8) and Tumor Necrosis Factor α (TNF-α) [7]. However the expression of Interleukin-10 (IL-10) secreted by microglia exposed to Aβ [8] and detected in cerebrospinal fluid (CSF) of patients with AD [9] suggests a homeostatic immune activity. An early primary feature of AD is cognitive deficit [10] that has been associated to increased expression of IL-12 [11]; this observation remains controversial [12]. Brain sections of AD patients show modifications in sialic acid expression in lesion areas [13]. However the the study of abnormal glycosylation patterns has only been focused on representative proteins of AD such as Beta secretase-1 (BACE-1), Amyloid-β Precursor Protein (AβPP), Transferrin (Tf), Tau and acetylcolinesterase (AChE) [14]. Low serum levels of sialyltransferases have also been described in AD [15] as well as alterations of AβPP O-glycosilation of endothelial cells exposed to Aβ [16]. The endothelial cell exposed to stress molecules such as Aβ, ROS, Low-Density Lipoprotein (LDL), or TNF-α secrete pro-inflammatory cytokines [17] amongst many other molecules [16]. Endothelial cells exposed to Aβ1-42 increase the secretion of nitric oxide (NO) [17] due to an increased activity of endothelial nitric oxide synthase [18]. The expression of inducible nitric oxide synthase (iNOS) has been seen in post mortem brain sections of the temporal cortex of AD patients [19], but its presence has not been determined in the serum of patients with mild cognitive impairment (MCI), that is, the stage is previous to the full-blown AD dementia [20]. The largest concentration of serum Aβ is found in the MCI stage [21] of AD progression. The aim of this work is to evaluate serum iNOS concentration in patients with MCI and to determine if it is associated with an increase in serum pro-inflammatory cytokines and alterations in sialylation of serum proteins. 2. Material and Methods 2.1. Study Group Our study was made with 46 healthy control inpiduals (male = 23, female = 23) over 55 years of age without dementia, neurology’s disease or auto immune’s disease, directed through the “Vida digna” association and the department of Neuropsychology of the Instituto Nacional de Neurología y Neurocirugía “Manuel Velasco Suarez”; ten patients with MCI (male = 5, female = 5) diagnosed one year earlier according to Petersen’s criteria [22]; and nineteen patients with probable AD dementia established 3 - 4 years earlier (male = 7, female = 12) who underwent extensive clinical, cognitive and biochemical evaluations according to the revised criteria established by the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease on 2011 [20] [22] [23]. An Mini-mental state examination (MMSE) test [23] (Impairment L. M. Castillo et al. 101 score ≤ 23) [24], was performed in each inpidual prior to the sample collection. All the inpiduals included in this study were informed of the aims of the study and the possible risks after which they all signed an informed consent form previously approved by the Ethic’s and Bioethic Committee of the Instituto Nacional de Neurologia y Neurocirugia “Manuel Velasco Suarez”. All clinical files are kept at the department of clinical archives at the Instituto Nacional de Neurología y Neurocirugía “Manuel Velasco Suarez” and are available upon request by a certified scientist. 2.2. Blood Samples A 5 ml blood sample was obtained via venipuncture using a Vacutainer collection tube without anticoagulant. The blood sample was left to rest at room temperature for 15 min followed by a 10 min centrifugation at 2000 rpm in a Beckman clinical centrifuge. At the end the serum was collected and kept at −70˚C before being used. 2.3. Electrophoresis and Western Blot Fifty µl (6.9 g/dL protein) of serum (Control = 9, MCI = 4, AD = 7; chosen using simple random sampling, were depleted of albumin with Pure Proteome TM Albumin Magnetic Beads (Millipore Corporation, Billerica, MA, USA) following the manufacturer’s instruction. A 40 µl (3.36 g/dL protein) volume was recovered after the procedure and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) in 10% gels at 120 V and 4˚C for 2 hrs was run using 4 µl (80 µg protein) of the albumin-free serum sample. At the end the proteins in the gel were transferred to polyvinylidene difluoride (PVDF) membrane using the Bio-Rad semi-dry transfer chamber. Before use, the membrane stained with Ponceau’s red to corroborate that an appropriate protein transfer had been obtained; afterwards the membrane was washed thrice in water and PVDF membrane was blocked with blocking buffer (PBS pH 7.4/ BSA 3% ) overnight at 4˚C. As soon as the blocking buffer was retired, the membrane was washed thrice in PBS pH 7.4/tween 20 0.05% before incubating it with polyclonal anti-iNOS antibody (Santa Cruz, Biotechn. CA, USA) 1:100, anti-Aβ (1:1000) (A3356, Sigma-Aldrich, St. Louis, Missouri; USA), and SNA (Sambucus nigra) lectin (Vector Labs, Burlingame, CA; USA) 1:20 in PBS/3% BSA overnight at 4˚C, afterwards the membrane was washed twice with PBS/0.01% tween 20 and incubated with an IgG anti-rabbit or anti-mouse secondary antibody conjugated with horseradish peroxidase (HRP) (Jackson Immuno Research, West Grove, PA; USA) 1:1000 or ExtrAvidin-Perox™ (Sigma-Aldrich, St. Louis, Missouri; USA) 1:2500, for the lectin assays, in PBS pH 7.4/BSA 3%/tween 20 0.05% for 30 minutes at room temperature. Transferrin, used as loading control, was detected with an anti-transferrin antibody (GenTex, Irvine, Ca, USA); in order to normalize load charge, transferrin concentration in the gel was determined by analyzing density by mean of evaluating dots per point (dpp)/band using GelQuant.NET 1.8.2 software (Biochemlabsolutions, University of California, San Franciso. USA). The reaction was revealed with the Super Signal West Dura Extended Duration Substrate (Thermo Scientific, Waktham, Massachussetts; USA) and density analysis of the signals was evaluated using the GelQuant.NET 1.8.2 software (Biochemlabsolutions, University of California, San Franciso. USA) that transforms the image into an 8-bit image; the program then calculates the intensity of the stained area measuring the amount of pixels/square inch and converts the readings into a white to black numerical value, expressed as dots per point (dpp). Apolipoproteins E (ApoE) genotype was determined by western blot analysis using Biolegend’s mouse polyclonal antibodies (clone 5G7 for E4, and clone 3C2 for E2); ApoE3 was detected with a rabbit polyclonal antibody (clone 27543, Abcam, San Francisco, CA). The procedure was identical to that already described in the above paragraphs. 2.4. Nitrites Were quantified by the method of Griess using the microplate Griess Reagent System (Promega Corporation, Madison WI, USA) that includes its own standard curve. Each sample was read in the Multiskan EX Thermo ELISA reader (Thermo Sci., USA). 2.5. Serum Interleukins TNF-α, IL-1β, IL-6, IL-8, IL-10 and IL-12 were determined by Enzyme-Linked Immuno Sorbent Assay (ELISA) method in each sample using commercially available kits (ELISA MAX Deluxe, BioLegend, San Diego, CA, L. M. Castillo et al. 102 USA) and following the manufacturer’s instruction. Micro plates were read using Multiskan EX Thermo (Thermo Scientific, Waktham, Massachussetts; USA). 2.6. Statistical Analysis Data was analyzed by an Analysis of Variance (ANOVA) test, followed by a post hoc test using the Turkey’s test. Pearson’s correlation coefficient was also used to convey the impact of the MMSE test and the serum parameters. All the statistical analysis was performed with GraphPad Prism 6.0 software (San Diego, CA, USA). All values are expressed as the mean ± SD. Differences were considered statistically significant when p < 0.05. 3. Results Our study group comprised 75 inpiduals, 61.33% of them were age-matched controls; the problem group was separated into the MCI group, that represented 13.33% of the study group, and the AD group that represented 25.33% of total the study group. Sociodemographic features are shown in Table 1. There was a highly significant increase in iNOS expression, as determined by the dpp values obtained in the western blot membranes, in the AD patients (ANOVA p = 0.0114) and a significant tendency of MCI patients in relation to control group (Student t p = 0.033) (Figure 1). Despite this the serum concentration of nitrites was significantly higher in the MCI patients 8.116 (µMol/l) in comparison to 4.286 (µMol/l) in the control group and 4.071 (µMol/l) in the AD group (p < 0.0001) (Figure 2). The relevance of the result is established when the results of the MMSE test were compared to the serum nitrite values. We found that the correlation between both was r = 0.6952, r 2 = 0.4833 and p = 0.0003, which in itself is not very high, it points towards a clear tendency where increased serum nitrates values might represent damage associated with early stages of AD development, that is, mild cognitive impairment (Figure 3). When pro-inflammatory cytokines were evaluated it surprised us to detect that the only two cytokines that were increased in patients with mild cognitive impairment and AD were TNF-α and IL-1β; the difference between them was not significant although in comparison to the control group it was highly significant (p < 0.0001 for IL-1β and p < 0.001 for TNF-α). Despite the seeming no difference between them, we found a predisposition for serum TNF to be higher in MCI patients than in AD patients. Not surprisingly interleukin-6 and interleukin-8 concentrations were significantly higher in AD patients in comparison to controls and MCI patients (p < 0.0001 and 0.0036, respectively) (Figure 4). Interestingly the serum concentration of interleukin-10 and interleukin-12 was almost identical, in the three groups (p = 0.7876 and 0.5007, respectively). As far as the staining intensity with the SNA lectin we also observed a major number of SNA + proteins in MCI serum samples than in AD samples but interestingly, the stain intensity for both was also greater than the control serum samples (Figure 5(a) and Figure 5(b)). Additionally, we found detectable levels of Aβ (4 kDa) only in three of the serum samples of the AD group (Figure 5(c)); the absence of Aβ in the remaining serum samples of the control and MCI groups was evident; this differences could be associated to the clinical stage of the disease as all the MCI patients were recently diagnosed and the AD patients with negative Aβ serum sample were catalogued as early or initial. Table 1. Descriptive analysis of the population analyzed. Mild Cognitive Impairment (MCI) and Alzheimer’s Disease (AD) * Right handed/Left handed. The p value represents the results of ANOVA analysis ± standard deviation. Statistical analysis of population. Control MCI AD p value Sample size 46 10 19 Mean age (years) 65 ± 7.24 66 ± 3.7 77 ± 5.8 Gender (male/female) 23/23 5/5 7/12 0.0036 (for AD) Laterality* 39/7 10/0 19/0 Mean education (years) 14 10 7.68 <0.0001 Serum total proteins (g/dL) 6.88 ± 1.56 6.79 ± 1.05 6.796 ± 1.47 0.6716 L. M. Castillo et al. 103 Figure 1. Comparative Western Blot density analysis of inducible Nitric Oxide Synthase (iNOS) in Control, Mild Cognitive Impairment (MCI) and Alzheimer’s Disease (AD) groups, and density of transferrin as load control. Control (lanes 1, 2, 3), MCI (lanes 4, 5, 6) and AD (lanes 7, 8, 9). Results are expressed as the mean ± standard deviation of the mean. iNOS ANOVA p =< 0.0001, Transferrin ANOVA p = 0.1459; * = p ≤ 0.05; **p = ≤ 0.001 dpp (dots per point). Figure 2. Serum levels of nitrites in control, Mild Cognitive Impairment (MCI) and Alzheimer’s Disease (AD) groups. Results are expressed as the mean ± standard deviation of the mean * p < 0.0001; NO (Nitric Oxide). ApoE alleles results in 9 AD patients, 4 MCI and 9 controls showed that eight AD patients were ApoE 4/4, and the remaining one was ApoE3/4; 3 of the MCI patients were 3/4 and one was 3/3. Seven controls were 2/3 whereas the remaining two were 3/3. 4. Discussion The characteristics of the population analyzed are in agreement to descriptions made in similar population stu- L. M. Castillo et al. 104 Figure 3. Correlation between MMSE (Mini-mental state examination) score and concentration of serum nitrite in Mild Cognitive Impairment (MCI) and Alzheimer Disease groups (r = 0.58, p = 0.0187). Figure 4. Serum level of interleukin in Mild Cognitive Impairment (MCI) and Alzheimer’s disease (AD) groups. TNF-alpha (Tumor Necrosis Factor-alpha), IL-1β (Interleukin-1β), IL-6 (Interleukin-6), IL-8 (Interleukin-8), IL-10 (Interleukin-10), IL-12 (Interleukin-12). Results are expressed as the mean ± standard deviation of the mean, * p < 0.05; **p < 0.001; ***p < 0.0001 L. M. Castillo et al. 105 (a) (b) (c) Figure 5. Western blot staining pattern of albumin-free sera stained with SNA lectin that recognizes O-glycans ((a) and (b)). In the figure A: Control samples (lanes 1-4), MCI samples (lanes 5 - 8), B: Control samples (lanes 1 - 4) and AD samples (lanes 5 - 11). Western blot staining amyloid-β of albumin-free sera (c). dies and reviews [25]-[27]. We are well aware that it will be ideal to follow up our patients in order to determine their decline, nevertheless all our AD patients are diagnosed and systematically evaluated at the Instituto for at least 3 - 4 years previous to their inclusion in this study. Inducible NOS, eNOS and NO regulate the inflammatory response [17], but endotelial Nitric Oxide Synthase (eNOS) also regulate the expression of the AβPP and BACE-1 an enzyme that cleaves AβPP [28]. It has been shown that the serum concentration of Aβ1-42 [23] is increased in MCI [29] and that iNOS stimulates endothelial cells to produce TNF-α and IL-1β in the presence of Aβ [30]. We find an increase in iNOS in MCI patients probably secondary to the high Aβ concentration, but it is well recognized that in the more advanced phase of the Alzheimer’s disease, the concentration of Aβ is almost negligible [31]. iNOS expression is, as expected, above that determined in MCI patients, the reasons can be that Aβ remains in the endothelial cells mitochondria or endoplasmic reticulum in the absence of serum Aβ [32] or that the damage to the endothelial cells during the MCI phase persists a possibility that needs further investigation. It has been shown that endothelial cells cleave the Aβ1-42 inside the cytoplasm to produce Aβ1-40 [18]; the latter is less toxic to the endothelial cell and this can be considered as a defense mechanism that is probably altered in AD patients. We find an increase in serum nitric oxide in the MCI patients and a decrease in AD. This is probably the consequence of the increased iNOS expression that we observe in the MCI patients, interestingly patients with AD show lower serum concentrations of NO [30] [31] [33] [34], which are not secondary to a decrease or disappearance of iNOS. It is possible that the lack of arginine that has been reported in AD patients [35] is the cause of such a decrease. The directly proportional correlation between MMSE score and levels of nitric oxide has only been described in cerebrospinal fluid in AD patients with severe damage [36]. We find an identical correlation when MMSE score and serum nitric oxide are evaluated. Nitric oxide is produced and secreted into CSF by neurons via neuronal Nitric Oxide Synthase (nNOS) and iNOS [37]; it has been shown that nitric oxide synthases respond to different hormones and peptides [38] and that a damaged endothelium induces the expression of iNOS [39]. Whether the continuous damage to the neurons in AD behaves similarly is unknown but a major player in AD is the role of the cerebral vascular endothelium, which is poorly understood. The study of serum interleukins so far has not included the MCI stage of the disease [12]. In our study, we find increased levels of TNF-α and IL-1β in MCI patients. TNF-α, and IL-1β are pro-inflammatory cytokine [40]. The increase of pro-inflammatory cytokines is related to an increase of iNOS, the latter stimulates the endothelial cell synthesis and secretion of TNF-α and IL-1β [30]. This apparently enhanced selective secretion of only two of the many pro-inflammatory cytokines can be related to “low endothelial damage”. This type of selective damage has been described in endothelium exposed to irradiation [41] and small amounts of ROS [42]. Nitric oxide is a reactive oxygen specie and our results show that in AD, the concentration is low. The serum inflammatory response is similar to reports of brain sections of AD [43]. The syalilation pattern in total and albumin-free serum proteins was evaluated with the SNA lectin (α-2,6) [44]. Both patterns were increased in AD and MCI samples, but the differences between them was significantly L. M. Castillo et al. 106 higher in AD samples. It has been shown that amyloid alters the syalilation pattern in the CA1 and DG subfields of the hippocampus [45] therefore the increased serum Aβ levels in MCI and AD patients [21] could explain the modification of serum proteins sialylation. Our results also showed a serum Aβ band (4 kDa) in some AD serum samples (Figure 5(c)). Glycosylation alterations have been widely reported in AD cerebral biopsies [13] [46], especially in brain endothelial cells exposed to Aβ [16]. In our study, the changes in the sialylation pattern can be related to the increase of TNF-α, IL-1 [47] or NO [48] as well as to the exposition of endothelial cells to serum Aβ. It has been shown that serum Aβ concentrations are higher in MCI in comparison to AD serum samples [21]. Endothelial cells show Aβ deposits in the reticulum and mitochondria [32] although the entrance mechanism remains to be determined it is possible that Aβ maintained in the cell reticulum could be the origin of these alterations since reticulum is the major site in the process of glycosilation [5]. ApoE allele ε4, not ε2 or ε3, is linked to late-onset Alzheimer’s disease and atherosclerosis [49]. Our IL-1, IL-6 and TNF-α results are in complete concordance with the results of Graybeal J.J. [50] that showed that IL-1β activity is altered by human ApoE ε4 alleles and Gale SC [51] that showed that ApoE ε4 alleles enhances innate immune responses as a consequence of Toll-like receptor (TLR) activation and TNF-α and IL-6 secretion. ApoE 4/4 haplotype is strongly associated to AD and MCI [52] but the ApoE 3/4 haplotype has also been detected in 20% - 30% of AD patients; similarly although the majority of elder people with no signs or symptoms of AD or MCI carry the 2/3 hapolotype there is a strong percentage of healthy inpiduals with the 3/3 hapolotype. Although our study evaluated small patient groups to provide meaningful data, our results clearly demonstrate that the clinically considered mid-phase of Alzheimer’s disease progression is related to early and not severe but continuous damage to the endothelium that is represented by the selective increase in certain pro-inflammatory cytokines, modification in the sialylation pattern of serum proteins and consequently the decrease in superior cerebral functions as determined by the MMSE test. The ApoE alleles results are consistent with those reported in the literature. We propose that a serum abnormal profile comprised by pro-inflammatory cytokines, iNOS and nitric oxide concentration, changes in Sambucus nigra serum proteins reactivit, and ApoE 3 or 4 haplotypes might be helpful in the early diagnosis of MCI, a stage in the natural history of AD that remains largely uncomprehended. Acknowledgements Castillo L.M. is a postgraduate student at the Biological Sciences Postgraduate Course, Universidad Nacional Autónoma de México, and a recipent of CONACyT-scholarship 288676. This study was partially funded by PAPIIT-IN211113 grant, UNAM, Mexico. References [1] Broussard, L., Myers, R. and Lemoine, J. (2009) Preparing Pediatric Nurses: The Role of Simulation-Based Learning. Issues in Comprehensive Pediatric Nursing, 32, 4-15. http://dx.doi.org/10.1080/01460860802610178 [2] Heneka, M.T., Kummer, M.P. and Latz, E. (2014) Innate Immune Activation in Neurodegenerative Disease. Nature Reviews Immunology, 14, 463-477. http://dx.doi.org/10.1038/nri3705 [3] Monsonego, A., Imitola, J., Zota, V., Oida, T. and Weiner, H.L. (2003) Microglia-Mediated Nitric Oxide Cytotoxicity of T Cells Following Amyloid β-Peptide Presentation to Th1 Cells. Journal of immunology, 171, 2216-2224. http://dx.doi.org/10.4049/jimmunol.171.5.2216 [4] Streit, W.J., Walter, S.A. and Pennell, N.A. (1999) Reactive Microgliosis. Progress in Neurobiology, 57, 563-581. http://dx.doi.org/10.1016/S0301-0082(98)00069-0 [5] Schedin-Weiss, S., Winblad, B. and Tjernberg, L.O. (2014) The Role of Protein Glycosylation in Alzheimer Disease. FEBS Journal, 281, 46-62. http://dx.doi.org/10.1111/febs.12590 [6] Tan, J., Town, T., Suo, Z., Wu, Y., Song, S., Kundtz, A., et al. (1999) Induction of CD40 on Human Endothelial Cells by Alzheimer’s β-Amyloid Peptides. Brain Research Bulletin, 50, 143-148. http://dx.doi.org/10.1016/S0361-9230(99)00122-7 [7] Rubio-Perez, J.M. and Morillas-Ruiz, J.M. (2012) A Review: Inflammatory Process in Alzheimer’s Disease, Role of Cytokines. Scientific World Journal, 2012, Article ID: 756357. http://dx.doi.org/10.1100/2012/756357 [8] Szczepanik, A.M., Funes, S., Petko, W. and Ringheim, G.E. (2001) IL-4, IL-10 and IL-13 Modulate a β(1-42)-Induced Cytokine and Chemokine Production in Primary Murine Microglia and a Human Monocyte Cell Line. Journal of Neu- L. M. Castillo et al. 107 roimmunology, 113, 49-62. http://dx.doi.org/10.1016/S0165-5728(00)00404-5 [9] Rota, E., Bellone, G., Rocca, P., Bergamasco, B., Emanuelli, G. and Ferrero, P. (2006) Increased Intrathecal TGF-β1, But Not IL-12, IFN-Gamma and IL-10 Levels in Alzheimer’s Disease Patients. Neurological Sciences: Official Journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology, 27, 33-39. http://dx.doi.org/10.1007/s10072-006-0562-6 [10] Backman, L., Jones, S., Berger, A.K., Laukka, E.J. and Small, B.J. (2004) Multiple Cognitive Deficits during the Transition to Alzheimer’s Disease. Journal of Internal Medicine, 256, 195-204. http://dx.doi.org/10.1111/j.1365-2796.2004.01386.x [11] Vom Berg J, Prokop S, Miller KR, Obst J, Kalin RE, Lopategui-Cabezas I, et al. Inhibition of IL-12/IL-23 signaling Vom Berg, J., Prokop, S., Miller, K.R., Obst, J., Kalin, R.E., Lopategui-Cabezas, I., et al. (2012) Inhibition of IL-12/ IL-23 Signaling Reduces Alzheimer’s Disease-Like Pathology and Cognitive Decline. Nature Medicine, 18, 1812-1819. http://dx.doi.org/10.1038/nm.2965 [12] Swardfager, W., Lanctot, K., Rothenburg, L., Wong, A., Cappell, J. and Herrmann, N. (2010) A Meta-Analysis of Cytokines in Alzheimer’s Disease. Biological Psychiatry, 68, 930-941. http://dx.doi.org/10.1016/j.biopsych.2010.06.012 [13] Guevara, J., Espinosa, B., Zenteno, E., Vazguez, L., Luna, J., Perry, G., et al. (1998) Altered Glycosylation Pattern of Proteins in Alzheimer Disease. Journal of Neuropathology and Experimental Neurology, 57, 905-914. http://dx.doi.org/10.1097/00005072-199810000-00003 [14] Marklová, E.A.Z. and Vališ, M. (2012) Microheterogeneity of Some Serum Glycoproteins in Neurodegenerative Diseases. Journal of the Neurological Sciences, 314, 20-25. http://dx.doi.org/10.1016/j.jns.2011.11.006 [15] Maguire, T.M., Gillian, A.M., O’Mahony, D., Coughlan, C.M., Dennihan, A. and Breen, K.C. (1994) A Decrease in Serum Sialyltransferase Levels in Alzheimer’s Disease. Neurobiology of Aging, 15, 99-102. http://dx.doi.org/10.1016/0197-4580(94)90149-X [16] Kitazume, S., Tachida, Y., Kato, M., Yamaguchi, Y., Honda, T., Hashimoto, Y., et al. (2010) Brain Endothelial Cells Produce Amyloid β from Amyloid Precursor Protein 770 and Preferentially Secrete the O-Glycosylated Form. The Journal of Biological Chemistry, 285, 40097-40103. http://dx.doi.org/10.1074/jbc.M110.144626 [17] Town, T., Tan, J. and Mullan, M. (2001) CD40 Signaling and Alzheimer’s Disease Pathogenesis. Neurochemistry International, 39, 371-380. http://dx.doi.org/10.1016/S0197-0186(01)00044-4 [18] Rajadas, J., Sun, W., Li, H., Inayathullah, M., Cereghetti, D., Tan, A., et al. (2013) Enhanced Aβ1-40 Production in Endothelial Cells Stimulated with Fibrillar Aβ1-42. PLoS ONE, 8, e58194. http://dx.doi.org/10.1371/journal.pone.0058194 [19] Fernandez-Vizarra, P., Fernandez, A.P., Castro-Blanco, S., Encinas, J.M., Serrano, J., Bentura, M.L., et al. (2004) Expression of Nitric Oxide System in Clinically Evaluated Cases of Alzheimer’s Disease. Neurobiology of Disease, 15, 287-305. http://dx.doi.org/10.1016/j.nbd.2003.10.010 [20] McKhann, G.M., Knopman, D.S., Chertkow, H., Hyman, B.T., Jack Jr., C.R., Kawas, C.H., et al. (2011) The Diagnosis of Dementia Due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 7, 263-269. http://dx.doi.org/10.1016/j.jalz.2011.03.005 [21] Luis, C.A., Abdullah, L., Paris, D., Quadros, A., Mullan, M., Mouzon, B., et al. (2009) Serum β-Amyloid Correlates with Neuropsychological Impairment. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition, 16, 203-218. http://dx.doi.org/10.1080/13825580802411766 [22] Albert, M.S., DeKosky, S.T., Dickson, D., Dubois, B., Feldman, H.H., Fox, N.C., et al. (2011) The Diagnosis of Mild Cognitive Impairment Due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 7, 270-279. http://dx.doi.org/10.1016/j.jalz.2011.03.008 [23] Segal-Gidan, F., Cherry, D., Jones, R., Williams, B., Hewett, L., Chodosh, J., et al. (2011) Alzheimer’s Disease Management Guideline: Update 2008. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 7, e51-e59. [24] México SdS. Mini examen del estado mental. Normas oficiales mexicanas. [25] Sharp, E.S. and Gatz, M. (2011) Relationship between Education and Dementia: An Updated Systematic Review. Alzheimer Disease and Associated Disorders, 25, 289-304. http://dx.doi.org/10.1097/WAD.0b013e318211c83c [26] Vemuri, P., Lesnick, T.G., Przybelski, S.A., Knopman, D.S., Roberts, R.O., Lowe, V.J., et al. (2012) Effect of Lifestyle Activities on Alzheimer Disease Biomarkers and Cognition. Annals of Neurology, 72, 730-738. http://dx.doi.org/10.1002/ana.23665 [27] Chene, G., Beiser, A., Au, R., Preis, S.R., Wolf, P.A., Dufouil, C., et al. (2015) Gender and Incidence of Dementia in the Framingham Heart Study from Mid-Adult Life. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 11, 310-320. http://dx.doi.org/10.1016/j.jalz.2013.10.005 L. M. Castillo et al. 108 [28] Katusic, Z.S. and Austin, S.A. (2014) Endothelial Nitric Oxide: Protector of a Healthy Mind. European Heart Journal, 35, 888-894. http://dx.doi.org/10.1093/eurheartj/eht544 [29] Abdullah, L., Luis, C., Paris, D., Ait-Ghezala, G., Mouzon, B., Allen, E., et al. (2009) High Serum Aβ and Vascular Risk Factors in First-Degree Relatives of Alzheimer’s Disease Patients. Molecular Medicine, 15, 95-100. http://dx.doi.org/10.2119/molmed.2008.00118 [30] Faro, M.L., Fox, B., Whatmore, J.L., Winyard, P.G. and Whiteman, M. (2014) Hydrogen Sulfide and Nitric Oxide Interactions in Inflammation. Nitric Oxide, 41, 38-47. [31] Tamaoka, A., Fukushima, T., Sawamura, N., Ishikawa, K., Oguni, E., Komatsuzaki, Y., et al. (1996) Amyloid Beta Protein in Plasma from Patients with Sporadic Alzheimer’s Disease. Journal of the Neurological Sciences, 141, 65-68. http://dx.doi.org/10.1016/0022-510X(96)00143-8 [32] Soriano, F.X., Galbete, J.L. and Forloni, G. (2003) Effect of Beta-Amyloid on Endothelial Cells: Lack of Direct Toxicity, Enhancement of MTT-Induced Cell Death and Intracellular Accumulation. Neurochemistry International, 43, 251- 261. http://dx.doi.org/10.1016/S0197-0186(03)00008-1 [33] Lodeiro, M., Ibanez, C., Cifuentes, A., Simo, C. and Cedazo-Minguez, A. (2014) Decreased Cerebrospinal Fluid Levels of L-Carnitine in Non-Apolipoprotein e4 Carriers at Early Stages of Alzheimer’s Disease. Journal of Alzheimer’s Disease: JAD, 41, 223-232. [34] Sachdeva, R., Babbar, R., Puri, V., Agarwal, S. and Krishana, B. (2011) Correlation between Cognitive Functions and Nitric Oxide Levels in Patients with Dementia. Clinical EEG and Neuroscience, 42, 190-194. http://dx.doi.org/10.1177/155005941104200309 [35] Ravaglia, G., Forti, P., Maioli, F., Bianchi, G., Martelli, M., Talerico, T., et al. (2004) Plasma Amino Acid Concentrations in Patients with Amnestic Mild Cognitive Impairment or Alzheimer Disease. The American Journal of Clinical Nutrition, 80, 483-488. [36] Tohgi, H., Abe, T., Yamazaki, K., Murata, T., Isobe, C. and Ishizaki, E. (1998) The Cerebrospinal Fluid Oxidized NO Metabolites, Nitrite and Nitrate, in Alzheimer’s Disease and Vascular Dementia of Binswanger Type and Multiple Small Infarct Type. Journal of Neural Transmission, 105, 1283-1291. http://dx.doi.org/10.1007/s007020050131 [37] Barbaresi, P., Fabri, M. and Mensa, E. (2014) Characterization of NO-Producing Neurons in the Rat Corpus Callosum. Brain and Behavior, 4, 317-336. http://dx.doi.org/10.1002/brb3.218 [38] Batra, S., Iosif, C., Al-Hijji, J. and Larsson, I. (2003) Important Differences in Nitric Oxide Synthase Activity and Predominant Isoform in Reproductive Tissues from Human and Rat. Reproductive Biology and Endocrinology, 1, 10. http://dx.doi.org/10.1186/1477-7827-1-10 [39] Lowry, J.L., Brovkovych, V., Zhang, Y. and Skidgel, R.A. (2013) Endothelial Nitric-Oxide Synthase Activation Generates an Inducible Nitric-Oxide Synthase-Like Output of Nitric Oxide in Inflamed Endothelium. The Journal of Biological Chemistry, 288, 4174-4193. http://dx.doi.org/10.1074/jbc.M112.436022 [40] Lane, T. and Lachmann, H.J. (2011) The Emerging Role of Interleukin-1β in Autoinflammatory Diseases. Current Allergy and Asthma Reports, 11, 361-368. http://dx.doi.org/10.1007/s11882-011-0207-6 [41] Cervelli, T., Panetta, D., Navarra, T., Andreassi, M.G., Basta, G., Galli, A., et al. (2014) Effects of Single and Fractionated Low-Dose Irradiation on Vascular Endothelial Cells. Atherosclerosis, 235, 510-518. http://dx.doi.org/10.1016/j.atherosclerosis.2014.05.932 [42] Lubrano, V. and Balzan, S. (2014) LOX-1 and ROS, Inseparable Factors in the Process of Endothelial Damage. Free Radical Research, 48, 841-848. http://dx.doi.org/10.3109/10715762.2014.929122 [43] Liu, L. and Chan, C. (2014) The Role of Inflammasome in Alzheimer’s Disease. Ageing Research Reviews, 15C, 6-15. http://dx.doi.org/10.1016/j.arr.2013.12.007 [44] Peumans, W.J., Roy, S., Barre, A., Rouge, P., van Leuven, F. and van Damme, E.J. (1998) Elderberry (Sambucus nigra) Contains Truncated Neu5Ac(α-2,6)Gal/GalNAc-Binding Type 2 Ribosome-Inactivating Proteins. FEBS Letters, 425, 35-39. http://dx.doi.org/10.1016/S0014-5793(98)00193-8 [45] Limon, I.D., Mendieta, L., Diaz, A., Chamorro, G., Espinosa, B., Zenteno, E., et al. (2009) Neuroprotective Effect of Alpha-Asarone on Spatial Memory and Nitric Oxide Levels in Rats Injected with Amyloid-β25-35. Neuroscience Letters, 453, 98-103. http://dx.doi.org/10.1016/j.neulet.2009.02.011 [46] Espinosa, B., Guevara, J., Hernandez, P., Slomianny, M.C., Guzman, A., Martinez-Cairo, S., et al. (2003) Characterization of an O-Glycosylated Plaque-Associated Protein from Alzheimer Disease Brain. Journal of Neuropathology and Experimental Neurology, 62, 34-41. [47] Hanasaki, K., Varki, A., Stamenkovic, I. and Bevilacqua, M.P. (1994) Cytokine-Induced Beta-Galactoside Alpha-2,6- Sialyltransferase in Human Endothelial Cells Mediates Alpha 2,6-Sialylation of Adhesion Molecules and CD22 Ligands. The Journal of Biological Chemistry, 269, 10637-10643. L. M. Castillo et al. 109 [48] Ngoh, G.A., Watson, L.J., Facundo, H.T. and Jones, S.P. (2011) Augmented O-GlcNAc Signaling Attenuates Oxidative Stress and Calcium Overload in Cardiomyocytes. Amino Acids, 40, 895-911. http://dx.doi.org/10.1007/s00726-010-0728-7 [49] Lathe, R., Sapronova, A. and Kotelevtsev, Y. (2014) Atherosclerosis and Alzheimer-Diseases with a Common Cause? Inflammation, Oxysterols, Vasculature. BMC Geriatrics, 14, 36. http://dx.doi.org/10.1186/1471-2318-14-36 [50] Graybeal, J.J., Bozzelli, P.L., Graybeal, L.L., Groeber, C.M., McKnight, P.E., Cox, D.N., et al. (2015) Human ApoE epsilon4 Alters Circadian Rhythm Activity, IL-1β, and GFAP in CRND8 Mice. Journal of Alzheimer’s Disease: JAD, 43, 823-834. [51] Gale, S.C., Gao, L., Mikacenic, C., Coyle, S.M., Rafaels, N., Murray Dudenkov, T., et al. (2014) APOepsilon4 Is Associated with Enhanced in Vivo Innate Immune Responses in Human Subjects. The Journal of Allergy and Clinical Immunology, 134, 127-134. http://dx.doi.org/10.1016/j.jaci.2014.01.032 [52] Qiu, W.Q., Zhu, H., Dean, M., Liu, Z., Vu, L., Fan, G., et al. (2015) Amyloid-Associated Depression and ApoE4 Allele: Longitudinal Follow-Up for the Development of Alzheimer’s Disease. International Journal of Geriatric Psychiatry, In Press. http://dx.doi.org/10.1002/gps.4339
IL-1β, TNF-α and Sambucus nigra Reactive Serum Proteins as Biomarkers of Mild Cognitive Impairment and Alzheimer Disease Progression Luis Manuel Castillo1, Enrique Moreno1, Yaneth Rodríguez-Agudelo2, Mireya Chávez-Oliveros2, Zoila Trujillo3, Blanca Espinosa4, Emma Rodriguez-Maldonado5, Luis Felipe Montaño6, JorgeGuevara1 1 Departamento de Bioquímica, Facultad de Medicina, Universidad Nacional Autónoma de México, México City, México 2 Departamento de Neuropsicología, Instituto Nacional de Neurología y Neurocirugía, México City, México 3 Geriatra Instituto Nacional de Neurología y Neurocirugía, México City, México 4 Laboratorio de Bioquímica, Instituto Nacional de Enfermedades Respiratorias, México City, México 5 Departamento de Fisiología, Instituto Nacional de Cardiología, México City, México 6 Departamento de Biología Celular y Tisular, Facultad de Medicina, Universidad Nacional Autónoma de México, México City, México Received 14 October 2015; accepted 12 December 2015; published 15 December 2015 Copyright © 2015 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Amyloid-β (Aβ) can induce a chronic inflammatory immune response that is associated, amongst many others, to abnormal glycosylation, inducible nitric oxide synthase (iNOS) and nitric oxide (NO). The relation between development of Mild Cognitive Impairment (MCI) and Alzheimer’s disease progression and these serum markers has not been evaluated. Serum levels of iNOs, NO, TNF-α, IL-1β, IL-6, IL-8, IL-10, and IL-12 are determined with commercially available kits. Sialylation of albumin-free serum patterns is determined by Western blot analysis with Sambucus nigra (specific for sialic acid attached to terminal galactose in α2,6 linkage) lectin. Apolipoprotein E (ApoE) haplotype is determined by Western blot using specific anti-ApoE 2, 3 or 4 antibodies. A mini-mental state examination (MMSE) test is also performed in the 10 MCI patients, 19 Alzheimer’s disease (AD) patients and 46 healthy age-matched controls evaluated. The results show an increase of iNOS in MCI and AD but significantly higher NO concentrations are only found in MCI patients. TNF-α and IL-1β concentrations are the only significantly increased cytokines in MCI patients; no differences between control and MCI or AD patients are found in regard to the other cy- L. M. Castillo et al. 100 tokines. An abnormal MMSE test result only correlates with a decrease in serum NO concentration in MCI patients. The terminal sialic acid linkage pattern of serum proteins also shows highly significant differences between MCI and AD patient. ApoE3/4 or 4/4 haplotypes are characteristic of MCI and AD patients. Our results imply that increased serum TNF-α, IL-1β, iNOS, NO and alterations of serum proteins glycosylation patterns in adult inpiduals with an abnormal MMSE test may serve as an early biomarker of MCI and AD development. Keywords Alzheimer Disease, Mild Cognitive Impairment, Nitric Oxide, Inflammatory Cytokines, Glycosylation, Apolipoprotein E4 1. Introduction The majority of pathology alterations in Alzheimer’s disease (AD) have been associated with amyloid-β (Aβ) toxicity [1]. Aβ can activate the microglia and macrophages [2], both of which become amyloid presenting cells to T cells of extra cerebral origin [3]; this in turn favors the local liberation of pro-inflammatory interleukins and reactive oxygen species (ROS) [4], as well as modifications of the post-translation processes of proteins including glycosylation [5]. This modification is not exclusive of immune cells as in vitro activation of endothelial cells exposed to the Aβ peptide has been shown [6]. Brain sections of AD patients express pro-inflammatory interleukins, such as: Interleukin-1 β (IL-1β), Interleukin-6 (IL-6), Interleukin-8 (IL-8) and Tumor Necrosis Factor α (TNF-α) [7]. However the expression of Interleukin-10 (IL-10) secreted by microglia exposed to Aβ [8] and detected in cerebrospinal fluid (CSF) of patients with AD [9] suggests a homeostatic immune activity. An early primary feature of AD is cognitive deficit [10] that has been associated to increased expression of IL-12 [11]; this observation remains controversial [12]. Brain sections of AD patients show modifications in sialic acid expression in lesion areas [13]. However the the study of abnormal glycosylation patterns has only been focused on representative proteins of AD such as Beta secretase-1 (BACE-1), Amyloid-β Precursor Protein (AβPP), Transferrin (Tf), Tau and acetylcolinesterase (AChE) [14]. Low serum levels of sialyltransferases have also been described in AD [15] as well as alterations of AβPP O-glycosilation of endothelial cells exposed to Aβ [16]. The endothelial cell exposed to stress molecules such as Aβ, ROS, Low-Density Lipoprotein (LDL), or TNF-α secrete pro-inflammatory cytokines [17] amongst many other molecules [16]. Endothelial cells exposed to Aβ1-42 increase the secretion of nitric oxide (NO) [17] due to an increased activity of endothelial nitric oxide synthase [18]. The expression of inducible nitric oxide synthase (iNOS) has been seen in post mortem brain sections of the temporal cortex of AD patients [19], but its presence has not been determined in the serum of patients with mild cognitive impairment (MCI), that is, the stage is previous to the full-blown AD dementia [20]. The largest concentration of serum Aβ is found in the MCI stage [21] of AD progression. The aim of this work is to evaluate serum iNOS concentration in patients with MCI and to determine if it is associated with an increase in serum pro-inflammatory cytokines and alterations in sialylation of serum proteins. 2. Material and Methods 2.1. Study Group Our study was made with 46 healthy control inpiduals (male = 23, female = 23) over 55 years of age without dementia, neurology’s disease or auto immune’s disease, directed through the “Vida digna” association and the department of Neuropsychology of the Instituto Nacional de Neurología y Neurocirugía “Manuel Velasco Suarez”; ten patients with MCI (male = 5, female = 5) diagnosed one year earlier according to Petersen’s criteria [22]; and nineteen patients with probable AD dementia established 3 - 4 years earlier (male = 7, female = 12) who underwent extensive clinical, cognitive and biochemical evaluations according to the revised criteria established by the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease on 2011 [20] [22] [23]. An Mini-mental state examination (MMSE) test [23] (Impairment L. M. Castillo et al. 101 score ≤ 23) [24], was performed in each inpidual prior to the sample collection. All the inpiduals included in this study were informed of the aims of the study and the possible risks after which they all signed an informed consent form previously approved by the Ethic’s and Bioethic Committee of the Instituto Nacional de Neurologia y Neurocirugia “Manuel Velasco Suarez”. All clinical files are kept at the department of clinical archives at the Instituto Nacional de Neurología y Neurocirugía “Manuel Velasco Suarez” and are available upon request by a certified scientist. 2.2. Blood Samples A 5 ml blood sample was obtained via venipuncture using a Vacutainer collection tube without anticoagulant. The blood sample was left to rest at room temperature for 15 min followed by a 10 min centrifugation at 2000 rpm in a Beckman clinical centrifuge. At the end the serum was collected and kept at −70˚C before being used. 2.3. Electrophoresis and Western Blot Fifty µl (6.9 g/dL protein) of serum (Control = 9, MCI = 4, AD = 7; chosen using simple random sampling, were depleted of albumin with Pure Proteome TM Albumin Magnetic Beads (Millipore Corporation, Billerica, MA, USA) following the manufacturer’s instruction. A 40 µl (3.36 g/dL protein) volume was recovered after the procedure and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) in 10% gels at 120 V and 4˚C for 2 hrs was run using 4 µl (80 µg protein) of the albumin-free serum sample. At the end the proteins in the gel were transferred to polyvinylidene difluoride (PVDF) membrane using the Bio-Rad semi-dry transfer chamber. Before use, the membrane stained with Ponceau’s red to corroborate that an appropriate protein transfer had been obtained; afterwards the membrane was washed thrice in water and PVDF membrane was blocked with blocking buffer (PBS pH 7.4/ BSA 3% ) overnight at 4˚C. As soon as the blocking buffer was retired, the membrane was washed thrice in PBS pH 7.4/tween 20 0.05% before incubating it with polyclonal anti-iNOS antibody (Santa Cruz, Biotechn. CA, USA) 1:100, anti-Aβ (1:1000) (A3356, Sigma-Aldrich, St. Louis, Missouri; USA), and SNA (Sambucus nigra) lectin (Vector Labs, Burlingame, CA; USA) 1:20 in PBS/3% BSA overnight at 4˚C, afterwards the membrane was washed twice with PBS/0.01% tween 20 and incubated with an IgG anti-rabbit or anti-mouse secondary antibody conjugated with horseradish peroxidase (HRP) (Jackson Immuno Research, West Grove, PA; USA) 1:1000 or ExtrAvidin-Perox™ (Sigma-Aldrich, St. Louis, Missouri; USA) 1:2500, for the lectin assays, in PBS pH 7.4/BSA 3%/tween 20 0.05% for 30 minutes at room temperature. Transferrin, used as loading control, was detected with an anti-transferrin antibody (GenTex, Irvine, Ca, USA); in order to normalize load charge, transferrin concentration in the gel was determined by analyzing density by mean of evaluating dots per point (dpp)/band using GelQuant.NET 1.8.2 software (Biochemlabsolutions, University of California, San Franciso. USA). The reaction was revealed with the Super Signal West Dura Extended Duration Substrate (Thermo Scientific, Waktham, Massachussetts; USA) and density analysis of the signals was evaluated using the GelQuant.NET 1.8.2 software (Biochemlabsolutions, University of California, San Franciso. USA) that transforms the image into an 8-bit image; the program then calculates the intensity of the stained area measuring the amount of pixels/square inch and converts the readings into a white to black numerical value, expressed as dots per point (dpp). Apolipoproteins E (ApoE) genotype was determined by western blot analysis using Biolegend’s mouse polyclonal antibodies (clone 5G7 for E4, and clone 3C2 for E2); ApoE3 was detected with a rabbit polyclonal antibody (clone 27543, Abcam, San Francisco, CA). The procedure was identical to that already described in the above paragraphs. 2.4. Nitrites Were quantified by the method of Griess using the microplate Griess Reagent System (Promega Corporation, Madison WI, USA) that includes its own standard curve. Each sample was read in the Multiskan EX Thermo ELISA reader (Thermo Sci., USA). 2.5. Serum Interleukins TNF-α, IL-1β, IL-6, IL-8, IL-10 and IL-12 were determined by Enzyme-Linked Immuno Sorbent Assay (ELISA) method in each sample using commercially available kits (ELISA MAX Deluxe, BioLegend, San Diego, CA, L. M. Castillo et al. 102 USA) and following the manufacturer’s instruction. Micro plates were read using Multiskan EX Thermo (Thermo Scientific, Waktham, Massachussetts; USA). 2.6. Statistical Analysis Data was analyzed by an Analysis of Variance (ANOVA) test, followed by a post hoc test using the Turkey’s test. Pearson’s correlation coefficient was also used to convey the impact of the MMSE test and the serum parameters. All the statistical analysis was performed with GraphPad Prism 6.0 software (San Diego, CA, USA). All values are expressed as the mean ± SD. Differences were considered statistically significant when p < 0.05. 3. Results Our study group comprised 75 inpiduals, 61.33% of them were age-matched controls; the problem group was separated into the MCI group, that represented 13.33% of the study group, and the AD group that represented 25.33% of total the study group. Sociodemographic features are shown in Table 1. There was a highly significant increase in iNOS expression, as determined by the dpp values obtained in the western blot membranes, in the AD patients (ANOVA p = 0.0114) and a significant tendency of MCI patients in relation to control group (Student t p = 0.033) (Figure 1). Despite this the serum concentration of nitrites was significantly higher in the MCI patients 8.116 (µMol/l) in comparison to 4.286 (µMol/l) in the control group and 4.071 (µMol/l) in the AD group (p < 0.0001) (Figure 2). The relevance of the result is established when the results of the MMSE test were compared to the serum nitrite values. We found that the correlation between both was r = 0.6952, r 2 = 0.4833 and p = 0.0003, which in itself is not very high, it points towards a clear tendency where increased serum nitrates values might represent damage associated with early stages of AD development, that is, mild cognitive impairment (Figure 3). When pro-inflammatory cytokines were evaluated it surprised us to detect that the only two cytokines that were increased in patients with mild cognitive impairment and AD were TNF-α and IL-1β; the difference between them was not significant although in comparison to the control group it was highly significant (p < 0.0001 for IL-1β and p < 0.001 for TNF-α). Despite the seeming no difference between them, we found a predisposition for serum TNF to be higher in MCI patients than in AD patients. Not surprisingly interleukin-6 and interleukin-8 concentrations were significantly higher in AD patients in comparison to controls and MCI patients (p < 0.0001 and 0.0036, respectively) (Figure 4). Interestingly the serum concentration of interleukin-10 and interleukin-12 was almost identical, in the three groups (p = 0.7876 and 0.5007, respectively). As far as the staining intensity with the SNA lectin we also observed a major number of SNA + proteins in MCI serum samples than in AD samples but interestingly, the stain intensity for both was also greater than the control serum samples (Figure 5(a) and Figure 5(b)). Additionally, we found detectable levels of Aβ (4 kDa) only in three of the serum samples of the AD group (Figure 5(c)); the absence of Aβ in the remaining serum samples of the control and MCI groups was evident; this differences could be associated to the clinical stage of the disease as all the MCI patients were recently diagnosed and the AD patients with negative Aβ serum sample were catalogued as early or initial. Table 1. Descriptive analysis of the population analyzed. Mild Cognitive Impairment (MCI) and Alzheimer’s Disease (AD) * Right handed/Left handed. The p value represents the results of ANOVA analysis ± standard deviation. Statistical analysis of population. Control MCI AD p value Sample size 46 10 19 Mean age (years) 65 ± 7.24 66 ± 3.7 77 ± 5.8 Gender (male/female) 23/23 5/5 7/12 0.0036 (for AD) Laterality* 39/7 10/0 19/0 Mean education (years) 14 10 7.68 <0.0001 Serum total proteins (g/dL) 6.88 ± 1.56 6.79 ± 1.05 6.796 ± 1.47 0.6716 L. M. Castillo et al. 103 Figure 1. Comparative Western Blot density analysis of inducible Nitric Oxide Synthase (iNOS) in Control, Mild Cognitive Impairment (MCI) and Alzheimer’s Disease (AD) groups, and density of transferrin as load control. Control (lanes 1, 2, 3), MCI (lanes 4, 5, 6) and AD (lanes 7, 8, 9). Results are expressed as the mean ± standard deviation of the mean. iNOS ANOVA p =< 0.0001, Transferrin ANOVA p = 0.1459; * = p ≤ 0.05; **p = ≤ 0.001 dpp (dots per point). Figure 2. Serum levels of nitrites in control, Mild Cognitive Impairment (MCI) and Alzheimer’s Disease (AD) groups. Results are expressed as the mean ± standard deviation of the mean * p < 0.0001; NO (Nitric Oxide). ApoE alleles results in 9 AD patients, 4 MCI and 9 controls showed that eight AD patients were ApoE 4/4, and the remaining one was ApoE3/4; 3 of the MCI patients were 3/4 and one was 3/3. Seven controls were 2/3 whereas the remaining two were 3/3. 4. Discussion The characteristics of the population analyzed are in agreement to descriptions made in similar population stu- L. M. Castillo et al. 104 Figure 3. Correlation between MMSE (Mini-mental state examination) score and concentration of serum nitrite in Mild Cognitive Impairment (MCI) and Alzheimer Disease groups (r = 0.58, p = 0.0187). Figure 4. Serum level of interleukin in Mild Cognitive Impairment (MCI) and Alzheimer’s disease (AD) groups. TNF-alpha (Tumor Necrosis Factor-alpha), IL-1β (Interleukin-1β), IL-6 (Interleukin-6), IL-8 (Interleukin-8), IL-10 (Interleukin-10), IL-12 (Interleukin-12). Results are expressed as the mean ± standard deviation of the mean, * p < 0.05; **p < 0.001; ***p < 0.0001 L. M. Castillo et al. 105 (a) (b) (c) Figure 5. Western blot staining pattern of albumin-free sera stained with SNA lectin that recognizes O-glycans ((a) and (b)). In the figure A: Control samples (lanes 1-4), MCI samples (lanes 5 - 8), B: Control samples (lanes 1 - 4) and AD samples (lanes 5 - 11). Western blot staining amyloid-β of albumin-free sera (c). dies and reviews [25]-[27]. We are well aware that it will be ideal to follow up our patients in order to determine their decline, nevertheless all our AD patients are diagnosed and systematically evaluated at the Instituto for at least 3 - 4 years previous to their inclusion in this study. Inducible NOS, eNOS and NO regulate the inflammatory response [17], but endotelial Nitric Oxide Synthase (eNOS) also regulate the expression of the AβPP and BACE-1 an enzyme that cleaves AβPP [28]. It has been shown that the serum concentration of Aβ1-42 [23] is increased in MCI [29] and that iNOS stimulates endothelial cells to produce TNF-α and IL-1β in the presence of Aβ [30]. We find an increase in iNOS in MCI patients probably secondary to the high Aβ concentration, but it is well recognized that in the more advanced phase of the Alzheimer’s disease, the concentration of Aβ is almost negligible [31]. iNOS expression is, as expected, above that determined in MCI patients, the reasons can be that Aβ remains in the endothelial cells mitochondria or endoplasmic reticulum in the absence of serum Aβ [32] or that the damage to the endothelial cells during the MCI phase persists a possibility that needs further investigation. It has been shown that endothelial cells cleave the Aβ1-42 inside the cytoplasm to produce Aβ1-40 [18]; the latter is less toxic to the endothelial cell and this can be considered as a defense mechanism that is probably altered in AD patients. We find an increase in serum nitric oxide in the MCI patients and a decrease in AD. This is probably the consequence of the increased iNOS expression that we observe in the MCI patients, interestingly patients with AD show lower serum concentrations of NO [30] [31] [33] [34], which are not secondary to a decrease or disappearance of iNOS. It is possible that the lack of arginine that has been reported in AD patients [35] is the cause of such a decrease. The directly proportional correlation between MMSE score and levels of nitric oxide has only been described in cerebrospinal fluid in AD patients with severe damage [36]. We find an identical correlation when MMSE score and serum nitric oxide are evaluated. Nitric oxide is produced and secreted into CSF by neurons via neuronal Nitric Oxide Synthase (nNOS) and iNOS [37]; it has been shown that nitric oxide synthases respond to different hormones and peptides [38] and that a damaged endothelium induces the expression of iNOS [39]. Whether the continuous damage to the neurons in AD behaves similarly is unknown but a major player in AD is the role of the cerebral vascular endothelium, which is poorly understood. The study of serum interleukins so far has not included the MCI stage of the disease [12]. In our study, we find increased levels of TNF-α and IL-1β in MCI patients. TNF-α, and IL-1β are pro-inflammatory cytokine [40]. The increase of pro-inflammatory cytokines is related to an increase of iNOS, the latter stimulates the endothelial cell synthesis and secretion of TNF-α and IL-1β [30]. This apparently enhanced selective secretion of only two of the many pro-inflammatory cytokines can be related to “low endothelial damage”. This type of selective damage has been described in endothelium exposed to irradiation [41] and small amounts of ROS [42]. Nitric oxide is a reactive oxygen specie and our results show that in AD, the concentration is low. The serum inflammatory response is similar to reports of brain sections of AD [43]. The syalilation pattern in total and albumin-free serum proteins was evaluated with the SNA lectin (α-2,6) [44]. Both patterns were increased in AD and MCI samples, but the differences between them was significantly L. M. Castillo et al. 106 higher in AD samples. It has been shown that amyloid alters the syalilation pattern in the CA1 and DG subfields of the hippocampus [45] therefore the increased serum Aβ levels in MCI and AD patients [21] could explain the modification of serum proteins sialylation. Our results also showed a serum Aβ band (4 kDa) in some AD serum samples (Figure 5(c)). Glycosylation alterations have been widely reported in AD cerebral biopsies [13] [46], especially in brain endothelial cells exposed to Aβ [16]. In our study, the changes in the sialylation pattern can be related to the increase of TNF-α, IL-1 [47] or NO [48] as well as to the exposition of endothelial cells to serum Aβ. It has been shown that serum Aβ concentrations are higher in MCI in comparison to AD serum samples [21]. Endothelial cells show Aβ deposits in the reticulum and mitochondria [32] although the entrance mechanism remains to be determined it is possible that Aβ maintained in the cell reticulum could be the origin of these alterations since reticulum is the major site in the process of glycosilation [5]. ApoE allele ε4, not ε2 or ε3, is linked to late-onset Alzheimer’s disease and atherosclerosis [49]. Our IL-1, IL-6 and TNF-α results are in complete concordance with the results of Graybeal J.J. [50] that showed that IL-1β activity is altered by human ApoE ε4 alleles and Gale SC [51] that showed that ApoE ε4 alleles enhances innate immune responses as a consequence of Toll-like receptor (TLR) activation and TNF-α and IL-6 secretion. ApoE 4/4 haplotype is strongly associated to AD and MCI [52] but the ApoE 3/4 haplotype has also been detected in 20% - 30% of AD patients; similarly although the majority of elder people with no signs or symptoms of AD or MCI carry the 2/3 hapolotype there is a strong percentage of healthy inpiduals with the 3/3 hapolotype. Although our study evaluated small patient groups to provide meaningful data, our results clearly demonstrate that the clinically considered mid-phase of Alzheimer’s disease progression is related to early and not severe but continuous damage to the endothelium that is represented by the selective increase in certain pro-inflammatory cytokines, modification in the sialylation pattern of serum proteins and consequently the decrease in superior cerebral functions as determined by the MMSE test. The ApoE alleles results are consistent with those reported in the literature. We propose that a serum abnormal profile comprised by pro-inflammatory cytokines, iNOS and nitric oxide concentration, changes in Sambucus nigra serum proteins reactivit, and ApoE 3 or 4 haplotypes might be helpful in the early diagnosis of MCI, a stage in the natural history of AD that remains largely uncomprehended. Acknowledgements Castillo L.M. is a postgraduate student at the Biological Sciences Postgraduate Course, Universidad Nacional Autónoma de México, and a recipent of CONACyT-scholarship 288676. This study was partially funded by PAPIIT-IN211113 grant, UNAM, Mexico. References [1] Broussard, L., Myers, R. and Lemoine, J. (2009) Preparing Pediatric Nurses: The Role of Simulation-Based Learning. Issues in Comprehensive Pediatric Nursing, 32, 4-15. http://dx.doi.org/10.1080/01460860802610178 [2] Heneka, M.T., Kummer, M.P. and Latz, E. (2014) Innate Immune Activation in Neurodegenerative Disease. Nature Reviews Immunology, 14, 463-477. http://dx.doi.org/10.1038/nri3705 [3] Monsonego, A., Imitola, J., Zota, V., Oida, T. and Weiner, H.L. (2003) Microglia-Mediated Nitric Oxide Cytotoxicity of T Cells Following Amyloid β-Peptide Presentation to Th1 Cells. Journal of immunology, 171, 2216-2224. http://dx.doi.org/10.4049/jimmunol.171.5.2216 [4] Streit, W.J., Walter, S.A. and Pennell, N.A. (1999) Reactive Microgliosis. Progress in Neurobiology, 57, 563-581. http://dx.doi.org/10.1016/S0301-0082(98)00069-0 [5] Schedin-Weiss, S., Winblad, B. and Tjernberg, L.O. (2014) The Role of Protein Glycosylation in Alzheimer Disease. FEBS Journal, 281, 46-62. http://dx.doi.org/10.1111/febs.12590 [6] Tan, J., Town, T., Suo, Z., Wu, Y., Song, S., Kundtz, A., et al. (1999) Induction of CD40 on Human Endothelial Cells by Alzheimer’s β-Amyloid Peptides. Brain Research Bulletin, 50, 143-148. http://dx.doi.org/10.1016/S0361-9230(99)00122-7 [7] Rubio-Perez, J.M. and Morillas-Ruiz, J.M. (2012) A Review: Inflammatory Process in Alzheimer’s Disease, Role of Cytokines. Scientific World Journal, 2012, Article ID: 756357. http://dx.doi.org/10.1100/2012/756357 [8] Szczepanik, A.M., Funes, S., Petko, W. and Ringheim, G.E. (2001) IL-4, IL-10 and IL-13 Modulate a β(1-42)-Induced Cytokine and Chemokine Production in Primary Murine Microglia and a Human Monocyte Cell Line. Journal of Neu- L. M. Castillo et al. 107 roimmunology, 113, 49-62. http://dx.doi.org/10.1016/S0165-5728(00)00404-5 [9] Rota, E., Bellone, G., Rocca, P., Bergamasco, B., Emanuelli, G. and Ferrero, P. (2006) Increased Intrathecal TGF-β1, But Not IL-12, IFN-Gamma and IL-10 Levels in Alzheimer’s Disease Patients. Neurological Sciences: Official Journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology, 27, 33-39. http://dx.doi.org/10.1007/s10072-006-0562-6 [10] Backman, L., Jones, S., Berger, A.K., Laukka, E.J. and Small, B.J. (2004) Multiple Cognitive Deficits during the Transition to Alzheimer’s Disease. Journal of Internal Medicine, 256, 195-204. http://dx.doi.org/10.1111/j.1365-2796.2004.01386.x [11] Vom Berg J, Prokop S, Miller KR, Obst J, Kalin RE, Lopategui-Cabezas I, et al. Inhibition of IL-12/IL-23 signaling Vom Berg, J., Prokop, S., Miller, K.R., Obst, J., Kalin, R.E., Lopategui-Cabezas, I., et al. (2012) Inhibition of IL-12/ IL-23 Signaling Reduces Alzheimer’s Disease-Like Pathology and Cognitive Decline. Nature Medicine, 18, 1812-1819. http://dx.doi.org/10.1038/nm.2965 [12] Swardfager, W., Lanctot, K., Rothenburg, L., Wong, A., Cappell, J. and Herrmann, N. (2010) A Meta-Analysis of Cytokines in Alzheimer’s Disease. Biological Psychiatry, 68, 930-941. http://dx.doi.org/10.1016/j.biopsych.2010.06.012 [13] Guevara, J., Espinosa, B., Zenteno, E., Vazguez, L., Luna, J., Perry, G., et al. (1998) Altered Glycosylation Pattern of Proteins in Alzheimer Disease. Journal of Neuropathology and Experimental Neurology, 57, 905-914. http://dx.doi.org/10.1097/00005072-199810000-00003 [14] Marklová, E.A.Z. and Vališ, M. (2012) Microheterogeneity of Some Serum Glycoproteins in Neurodegenerative Diseases. Journal of the Neurological Sciences, 314, 20-25. http://dx.doi.org/10.1016/j.jns.2011.11.006 [15] Maguire, T.M., Gillian, A.M., O’Mahony, D., Coughlan, C.M., Dennihan, A. and Breen, K.C. (1994) A Decrease in Serum Sialyltransferase Levels in Alzheimer’s Disease. Neurobiology of Aging, 15, 99-102. http://dx.doi.org/10.1016/0197-4580(94)90149-X [16] Kitazume, S., Tachida, Y., Kato, M., Yamaguchi, Y., Honda, T., Hashimoto, Y., et al. (2010) Brain Endothelial Cells Produce Amyloid β from Amyloid Precursor Protein 770 and Preferentially Secrete the O-Glycosylated Form. The Journal of Biological Chemistry, 285, 40097-40103. http://dx.doi.org/10.1074/jbc.M110.144626 [17] Town, T., Tan, J. and Mullan, M. (2001) CD40 Signaling and Alzheimer’s Disease Pathogenesis. Neurochemistry International, 39, 371-380. http://dx.doi.org/10.1016/S0197-0186(01)00044-4 [18] Rajadas, J., Sun, W., Li, H., Inayathullah, M., Cereghetti, D., Tan, A., et al. (2013) Enhanced Aβ1-40 Production in Endothelial Cells Stimulated with Fibrillar Aβ1-42. PLoS ONE, 8, e58194. http://dx.doi.org/10.1371/journal.pone.0058194 [19] Fernandez-Vizarra, P., Fernandez, A.P., Castro-Blanco, S., Encinas, J.M., Serrano, J., Bentura, M.L., et al. (2004) Expression of Nitric Oxide System in Clinically Evaluated Cases of Alzheimer’s Disease. Neurobiology of Disease, 15, 287-305. http://dx.doi.org/10.1016/j.nbd.2003.10.010 [20] McKhann, G.M., Knopman, D.S., Chertkow, H., Hyman, B.T., Jack Jr., C.R., Kawas, C.H., et al. (2011) The Diagnosis of Dementia Due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 7, 263-269. http://dx.doi.org/10.1016/j.jalz.2011.03.005 [21] Luis, C.A., Abdullah, L., Paris, D., Quadros, A., Mullan, M., Mouzon, B., et al. (2009) Serum β-Amyloid Correlates with Neuropsychological Impairment. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition, 16, 203-218. http://dx.doi.org/10.1080/13825580802411766 [22] Albert, M.S., DeKosky, S.T., Dickson, D., Dubois, B., Feldman, H.H., Fox, N.C., et al. (2011) The Diagnosis of Mild Cognitive Impairment Due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 7, 270-279. http://dx.doi.org/10.1016/j.jalz.2011.03.008 [23] Segal-Gidan, F., Cherry, D., Jones, R., Williams, B., Hewett, L., Chodosh, J., et al. (2011) Alzheimer’s Disease Management Guideline: Update 2008. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 7, e51-e59. [24] México SdS. Mini examen del estado mental. Normas oficiales mexicanas. [25] Sharp, E.S. and Gatz, M. (2011) Relationship between Education and Dementia: An Updated Systematic Review. Alzheimer Disease and Associated Disorders, 25, 289-304. http://dx.doi.org/10.1097/WAD.0b013e318211c83c [26] Vemuri, P., Lesnick, T.G., Przybelski, S.A., Knopman, D.S., Roberts, R.O., Lowe, V.J., et al. (2012) Effect of Lifestyle Activities on Alzheimer Disease Biomarkers and Cognition. Annals of Neurology, 72, 730-738. http://dx.doi.org/10.1002/ana.23665 [27] Chene, G., Beiser, A., Au, R., Preis, S.R., Wolf, P.A., Dufouil, C., et al. (2015) Gender and Incidence of Dementia in the Framingham Heart Study from Mid-Adult Life. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 11, 310-320. http://dx.doi.org/10.1016/j.jalz.2013.10.005 L. M. Castillo et al. 108 [28] Katusic, Z.S. and Austin, S.A. (2014) Endothelial Nitric Oxide: Protector of a Healthy Mind. European Heart Journal, 35, 888-894. http://dx.doi.org/10.1093/eurheartj/eht544 [29] Abdullah, L., Luis, C., Paris, D., Ait-Ghezala, G., Mouzon, B., Allen, E., et al. (2009) High Serum Aβ and Vascular Risk Factors in First-Degree Relatives of Alzheimer’s Disease Patients. Molecular Medicine, 15, 95-100. http://dx.doi.org/10.2119/molmed.2008.00118 [30] Faro, M.L., Fox, B., Whatmore, J.L., Winyard, P.G. and Whiteman, M. (2014) Hydrogen Sulfide and Nitric Oxide Interactions in Inflammation. Nitric Oxide, 41, 38-47. [31] Tamaoka, A., Fukushima, T., Sawamura, N., Ishikawa, K., Oguni, E., Komatsuzaki, Y., et al. (1996) Amyloid Beta Protein in Plasma from Patients with Sporadic Alzheimer’s Disease. Journal of the Neurological Sciences, 141, 65-68. http://dx.doi.org/10.1016/0022-510X(96)00143-8 [32] Soriano, F.X., Galbete, J.L. and Forloni, G. (2003) Effect of Beta-Amyloid on Endothelial Cells: Lack of Direct Toxicity, Enhancement of MTT-Induced Cell Death and Intracellular Accumulation. Neurochemistry International, 43, 251- 261. http://dx.doi.org/10.1016/S0197-0186(03)00008-1 [33] Lodeiro, M., Ibanez, C., Cifuentes, A., Simo, C. and Cedazo-Minguez, A. (2014) Decreased Cerebrospinal Fluid Levels of L-Carnitine in Non-Apolipoprotein e4 Carriers at Early Stages of Alzheimer’s Disease. Journal of Alzheimer’s Disease: JAD, 41, 223-232. [34] Sachdeva, R., Babbar, R., Puri, V., Agarwal, S. and Krishana, B. (2011) Correlation between Cognitive Functions and Nitric Oxide Levels in Patients with Dementia. Clinical EEG and Neuroscience, 42, 190-194. http://dx.doi.org/10.1177/155005941104200309 [35] Ravaglia, G., Forti, P., Maioli, F., Bianchi, G., Martelli, M., Talerico, T., et al. (2004) Plasma Amino Acid Concentrations in Patients with Amnestic Mild Cognitive Impairment or Alzheimer Disease. The American Journal of Clinical Nutrition, 80, 483-488. [36] Tohgi, H., Abe, T., Yamazaki, K., Murata, T., Isobe, C. and Ishizaki, E. (1998) The Cerebrospinal Fluid Oxidized NO Metabolites, Nitrite and Nitrate, in Alzheimer’s Disease and Vascular Dementia of Binswanger Type and Multiple Small Infarct Type. Journal of Neural Transmission, 105, 1283-1291. http://dx.doi.org/10.1007/s007020050131 [37] Barbaresi, P., Fabri, M. and Mensa, E. (2014) Characterization of NO-Producing Neurons in the Rat Corpus Callosum. Brain and Behavior, 4, 317-336. http://dx.doi.org/10.1002/brb3.218 [38] Batra, S., Iosif, C., Al-Hijji, J. and Larsson, I. (2003) Important Differences in Nitric Oxide Synthase Activity and Predominant Isoform in Reproductive Tissues from Human and Rat. Reproductive Biology and Endocrinology, 1, 10. http://dx.doi.org/10.1186/1477-7827-1-10 [39] Lowry, J.L., Brovkovych, V., Zhang, Y. and Skidgel, R.A. (2013) Endothelial Nitric-Oxide Synthase Activation Generates an Inducible Nitric-Oxide Synthase-Like Output of Nitric Oxide in Inflamed Endothelium. The Journal of Biological Chemistry, 288, 4174-4193. http://dx.doi.org/10.1074/jbc.M112.436022 [40] Lane, T. and Lachmann, H.J. (2011) The Emerging Role of Interleukin-1β in Autoinflammatory Diseases. Current Allergy and Asthma Reports, 11, 361-368. http://dx.doi.org/10.1007/s11882-011-0207-6 [41] Cervelli, T., Panetta, D., Navarra, T., Andreassi, M.G., Basta, G., Galli, A., et al. (2014) Effects of Single and Fractionated Low-Dose Irradiation on Vascular Endothelial Cells. Atherosclerosis, 235, 510-518. http://dx.doi.org/10.1016/j.atherosclerosis.2014.05.932 [42] Lubrano, V. and Balzan, S. (2014) LOX-1 and ROS, Inseparable Factors in the Process of Endothelial Damage. Free Radical Research, 48, 841-848. http://dx.doi.org/10.3109/10715762.2014.929122 [43] Liu, L. and Chan, C. (2014) The Role of Inflammasome in Alzheimer’s Disease. Ageing Research Reviews, 15C, 6-15. http://dx.doi.org/10.1016/j.arr.2013.12.007 [44] Peumans, W.J., Roy, S., Barre, A., Rouge, P., van Leuven, F. and van Damme, E.J. (1998) Elderberry (Sambucus nigra) Contains Truncated Neu5Ac(α-2,6)Gal/GalNAc-Binding Type 2 Ribosome-Inactivating Proteins. FEBS Letters, 425, 35-39. http://dx.doi.org/10.1016/S0014-5793(98)00193-8 [45] Limon, I.D., Mendieta, L., Diaz, A., Chamorro, G., Espinosa, B., Zenteno, E., et al. (2009) Neuroprotective Effect of Alpha-Asarone on Spatial Memory and Nitric Oxide Levels in Rats Injected with Amyloid-β25-35. Neuroscience Letters, 453, 98-103. http://dx.doi.org/10.1016/j.neulet.2009.02.011 [46] Espinosa, B., Guevara, J., Hernandez, P., Slomianny, M.C., Guzman, A., Martinez-Cairo, S., et al. (2003) Characterization of an O-Glycosylated Plaque-Associated Protein from Alzheimer Disease Brain. Journal of Neuropathology and Experimental Neurology, 62, 34-41. [47] Hanasaki, K., Varki, A., Stamenkovic, I. and Bevilacqua, M.P. (1994) Cytokine-Induced Beta-Galactoside Alpha-2,6- Sialyltransferase in Human Endothelial Cells Mediates Alpha 2,6-Sialylation of Adhesion Molecules and CD22 Ligands. The Journal of Biological Chemistry, 269, 10637-10643. L. M. Castillo et al. 109 [48] Ngoh, G.A., Watson, L.J., Facundo, H.T. and Jones, S.P. (2011) Augmented O-GlcNAc Signaling Attenuates Oxidative Stress and Calcium Overload in Cardiomyocytes. Amino Acids, 40, 895-911. http://dx.doi.org/10.1007/s00726-010-0728-7 [49] Lathe, R., Sapronova, A. and Kotelevtsev, Y. (2014) Atherosclerosis and Alzheimer-Diseases with a Common Cause? Inflammation, Oxysterols, Vasculature. BMC Geriatrics, 14, 36. http://dx.doi.org/10.1186/1471-2318-14-36 [50] Graybeal, J.J., Bozzelli, P.L., Graybeal, L.L., Groeber, C.M., McKnight, P.E., Cox, D.N., et al. (2015) Human ApoE epsilon4 Alters Circadian Rhythm Activity, IL-1β, and GFAP in CRND8 Mice. Journal of Alzheimer’s Disease: JAD, 43, 823-834. [51] Gale, S.C., Gao, L., Mikacenic, C., Coyle, S.M., Rafaels, N., Murray Dudenkov, T., et al. (2014) APOepsilon4 Is Associated with Enhanced in Vivo Innate Immune Responses in Human Subjects. The Journal of Allergy and Clinical Immunology, 134, 127-134. http://dx.doi.org/10.1016/j.jaci.2014.01.032 [52] Qiu, W.Q., Zhu, H., Dean, M., Liu, Z., Vu, L., Fan, G., et al. (2015) Amyloid-Associated Depression and ApoE4 Allele: Longitudinal Follow-Up for the Development of Alzheimer’s Disease. International Journal of Geriatric Psychiatry, In Press. http://dx.doi.org/10.1002/gps.4339


